

Examples are glucose, fructose, glyceraldehydes, lactose, arabinose and maltose, except for sucrose.
#Non reducing anomeric carbon free
Satyanarayana and U.Reducing Sugar (biology definition): A sugar that serves as a reducing agent due to its free aldehyde or ketone functional groups in its molecular structure.
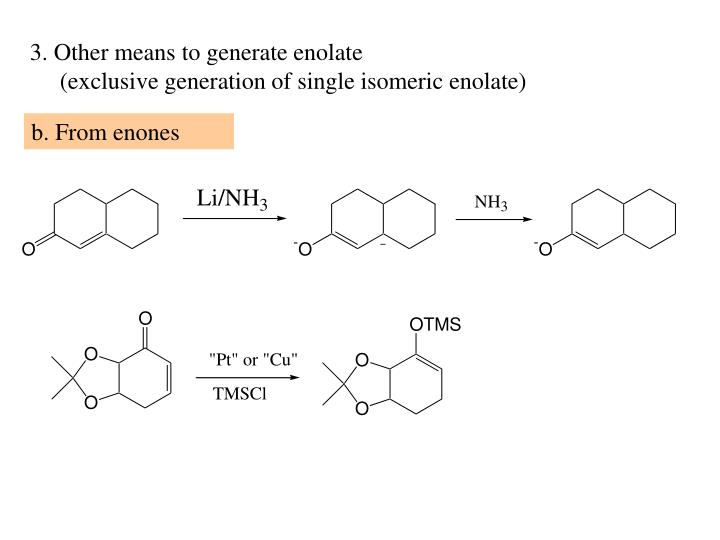
This is because it contains a free aldehyde group. However, non-reducing cannot reduce others. Therefore, they are named reducing sugars. Why are reducing and non-reducing ends of sugars named so?
The benedict’s and feeling solution test is used to confirm the reducing sugars. What test can be used to confirm a reduced sugar? There are examples of non-reducing sugars: What are five examples of non-reducing sugars?
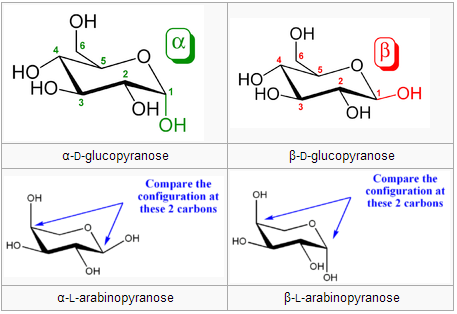
There are the following examples of reducing sugars: This is because it contains a free ketone group. Therefore, they do not reduce others.įructose is a reducing sugar. At the same time, the non-reducing sugars do not contain free aldehyde and ketone groups. The reducing sugars contain free aldehyde and ketone groups. How do you know if sugar is reducing or non-reducing? Mutarotation: The α,β Sugars Interconversion.Glycogen in the liver helps to maintain the level of glucose.Starch is used in the preservation of baked food.The cellulose act as the raw material for the formation of the cellulose acetate.Cellulose is used as a structural material to provide rigidity to plants.The uses of non-reducing sugars are the following: They are used as reducing agents in the chemical reactions.Reducing sugars lowers the risk of developing obesity and diabetes.In the food industry, the level of reduced sugars decides their quality.If we know the exact amount of glucose in our body, then we get an idea about the amount of insulin that a patient must be taken.Reducing sugars are the most abundant organic molecules found in nature. Uses of reducing and non-reducing sugars Reducing sugars Whereas non-reducing sugars do not show this property. The reducing sugars can form osazone by reacting with phenylhydrazine. While non reducing sugars does not give this test.ĬH 2OH(CHOH) 4CHO + 2CuO → CH 2OHCHOH 4COOH + Cu 2O (Red ppt) Osazone formation If the red color precipitates are formed, it confirms the presence of reducing sugars. In the Fehling test, a sample is first heated, and after that Fehling solution is added to it. If the color of the solution changes to green orange or red confirms the presence of reducing sugars in the food sample. If it changes to blue, it means that no reducing sugar is present. However, after ten to fifteen minutes note the color of the solution. It is dissolved in water and later benedict’s solution is added. In this test, first, we take the food sample. We can differentiate between reducing and non-reducing sugars by the following test: Benedict’s Test Test to distinguish reducing vs non-reducing sugars Note that, polysaccharides such as starch are non-reducing sugars. So, sucrose does not contain a free carbonyl group. Hence, the carbonyl groups of both monosaccharides participate in the glycosidic bond. This is because the combination of glucose and fructose forms it. They do not contain anomeric carbon attached to the hydroxyl (-OH) group.įor instance, sucrose is a disaccharide but it is a non-reducing sugar. Non-reducing sugarsĬarbohydrate which does not contains free aldehyde and ketone group is known as non-reducing sugars. Some disaccharides are also reducing sugars such as lactose. The anomeric carbon is that which is derived from the carbonyl group and contains two oxygen substituents.Īll monosaccharides are reducing sugars. However, reducing sugars contain free anomeric carbon. They can donate electrons to other compounds and cause the reduction of other compounds. Reducing sugars contain free aldehyde and ketone groups. What are reducing and non-reducing sugars? Reducing sugars They can be used as precursors in millard reaction Negative result with Tollen's reagent (no reaction)Ĭolor changes are observed in Benedict test Positive result with Tollen's reagent (forms a silver mirror) Sugars that do not have a free aldehyde or ketone group and cannot reduce other compoundsĬommon Examples: glucose, fructose, maltoseĬommon Examples: sucrose, lactose, cellulose Sugars that have a free aldehyde or ketone group and can reduce other compounds


 0 kommentar(er)
0 kommentar(er)
